Advocacy for Access to Nusinersen and Risdiplam

Advocacy for Access to Nusinersen and Risdiplam
Page last updated: 6th January 2025

The National Institute of Health and Care Excellence (NICE) approved Nusinersen (Spinraza) for funding by the NHS in England in July 2019 and for Risdiplam (Evrysdi) in August 2020. Both treatments have been funded via time limited Managed Access Agreements (MAA).
Open the tabs below to find out what is happening now…..
The December 2024 NICE committee meeting to discuss the clinical and cost effectiveness of these two drugs was postponed.
NICE listened to, and agreed with, the concerns raised by SMA UK, our partnerpatient organizations, SMA clinicians, and pharmaceutical companies about the External Assessment Group (EAG)’s report. The report was to critically evaluate the clinical and economic evidence for Spinraza and Risdiplam.
- They have asked that there is engagement with the patient and professional organisations to ensure the report’s validity.
- They are confident that this will lead to a more robust report.
- The report should be ready by the beginning of October 2025.
- They will then hold a further 1-month consultation.
- They anticipate that a first committee discussion will now be 11th November 2025.
When to will we know what NICE recommends?
About three weeks after the committee meeting NICE will send out their draft recommendations to all consultees. The patient groups and others experts involved will then have 20 working days to submit their comments before the recommendations are published.
If there are no appeals against the draft recommendations, the guidance will be published about 16 weeks after the final committee meeting.
It often takes more than one committee meeting for NICE to make a very final recommendation.
Over the past 2 years, 17 of 21 rare disease medicines (81%) that received an interim negative decision after the first committee meeting eventually received a positive recommendation. This was usually after a second or third committee meeting.
Wales and Northern Ireland have followed NICE’s guidance on access and are likely to follow the recommendations.
What did we say about the EAG report?
Working collaboratively, and in consultation with NICE, SMA UK led on a joint patient organisation response to the EAG report. We submitted our response in September. We expressed our concerns that the report did not:
- Consider real world evidence as carefully as evidence from clinical trials.
- Show an understanding that mobility outcomes only do not reflect the holistic impact that SMA has on daily living.
- Seem to recognise that in a rare disease like SMA, clinical trials will always be small and long-term data is limited. Therefore, continuing to collect and review real world data is essential.
- Seem to recognise that treatment outcomes can be individualised, therefore having a choice of treatments is essential.
- Value stabilisation of the condition as a hugely positive treatment outcome. And that this should be assessed as a highly effective outcome across the SMA population.
- Current measures of quality of life and treatment outcomes are not sensitive enough. They cannot capture the benefits of SMA treatment across its vast and varied spectrum. Therefore, real world data from patients should be carefully considered.
What did NICE say about the comments on the EAG report?
NICE was very concerned about the issues raised. The main ones they highlighted were:
- Stakeholders felt that the report demonstrated a lack of understanding of SMA and a lack of clinical input and validation
- A concern that not all the available data had been used to its full potential, including the data collected during the managed access period
- Concerns about the lack of detail about the economic modelling and that it wasn’t clear about the changes made.
These MAAs allowed access to these treatments on the condition that researchers would collect more information from patients and their clinicians on:
- how well the treatment works,
- how safe it is
- whether or not it is good value for money.
In 2019 – 2020, the NICE committee did not feel there was enough evidence from the clinical trials to answer these questions for these treatments.
This is why, if you have been prescribed either of these drugs, you will have been completing physio assessments. You will also have been answering questions and completing patient reported outcomes. These are used as evidence of clinical effectiveness.
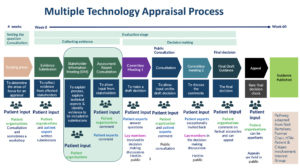
When two or more treatments are used for the same condition, NICE reviews them at the same time via a Multiple Technology Appraisal (MTA).
They look at:
- all the evidence that has been collected while the MAAs have been in place
- any new evidence from clinical trials
- international studies
They also invite any groups that have contact with, or are part of, the Patient and Clinical Community, to submit any evidence they hold. They are asked to give their views on the specific questions they are looking to answer.
You can see the list of these potential ‘stakeholders’ for this MTA on this NICE website page.
NICE also commission an external assessment group to review all the information This group examines all the evidence submitted by the different stakeholders. The results of this independent study are used to help the decision makers.
Clinical and Patient groups are able to read and comment on this report.
This webinar summarises the process, the role of the patient groups and includes our earlier call for action.
Video recorded: 17th February 2024
SMA UK, Muscular Dystrophy UK (MDUK) and TreatSMA are consultees to the appraisal committee. This means we can contribute to the decision-making process in a number of different ways.
August 2023 Scoping Consultation. We responded with MDUK to NICE’s invitation to review the background information for the review.
January 2024 Our Survey of Community Views. We agreed with clinicians and the pharmaceutical companies that the clinical evidence was not really reflecting the real-life experiences of treatment for those living with SMA. In particular, it was not capturing:
- the value of stabilisation for adults
- the importance of access for all no matter the severity of the condition.
We received responses from:
- 114 people living with SMA, bringing perspectives of both treatments being assessed
- 144 carers, sharing their invaluable thoughts and experiences.
April 2024 Joint patient groups submission and survey results sent to NICE. We led the unified voice of the three patient groups and the SMA Community.
Jointly we advocated that:
- In close consultation with individual’s specialist clinicians, treatment should be available for everyone living with SMA no matter the SMN copy number or clinical type.
- Measures used to assess quality of life and outcomes from treatments are not sensitive enough to capture the benefits that treatment for SMA brings across the vast and varied spectrum of the condition. Therefore, real world data from patients should be carefully considered.
March 2024 Patient and clinical experts.
NICE usually asks for nominations and selects two patient experts to give the patient perspective of the treatment at their committee meetings.
All three patient groups met with the chair of the committee to discuss why a wider representation is so important for this assessment.
The chair confirmed that he would allow three patient experts:
- Portia Thorman, representing children
- Lucy Frost, representing teenagers
- Andi Thorton, representing adults
An explanation of what happens if the committee does not recommend either treatment for NHS funding is written into the Managed Access Agreements. See the relevant extracts from the documents below:
Risdiplam (Evrysdi):
The full Risdiplam Managed Access Agreement
Nusinersen (Spinraza):
The full nusinersen Managed Access Agreement
We understand the anxiety this will bring to Spinraza-treated patients. At SMA UK we are doing all we can to retain NHS funding for both treatments, ensuring everyone living with SMA has the choice of available disease-modifying treatments to suit their individual needs. We will be advocating for full and equal access for all.
We asked Biogen UK for their comments. They said:
Biogen welcomes the action that NICE has taken to listen and respond to the issues within the External Assessment Group (EAG) report raised by Biogen and other stakeholders. It is essential that the new report reflects the complexity of SMA and that NICE and the EAG ensure the clinical and patient community’s views are heard.
We have now signed a variation to the original Managed Access Agreement (MAA) which extends the access arrangements to September 2026. This means access to nusinersen will continue for new and existing patients until final guidance is published following the appraisal outcome.
We hope this provides reassurance to patients and families. Please also be assured, Biogen is working very hard with NICE, NHS England and other partners to ensure the nusinersen NICE appraisal is positive and that we achieve broad access for as many patients as possible in the UK.
Our survey has now closed – thank you to everyone who submitted their responses.
Your views and experiences are always helpful for us. If you missed the survey and would still like to tell us why access to these treatments matter to you, please contact us in one of these ways:
- A written summary to office@smauk.org.uk
- Audio/video to WhatsApp 07759210888
- Use WeTransfer to send to office@smauk.org.uk
