Plastin 3 is a Potential Therapeutic Target for SMA
Plastin 3 is a Potential Therapeutic Target for SMA
Page last updated: 1st January 2013
In 2008, Plastin 3 was identified by Brunhilde Wirth and colleagues as a gender-specific protective modifier of SMA.
Very rarely, people can completely lack the survival motor neuron 1 (SMN1) gene and not develop SMA. This could be due to them having many copies of the SMN2 gene and therefore producing enough functional SMN protein to remain unaffected. Alternatively, the levels of other proteins can change to compensate for there being less SMN, which reduces the severity of SMA or even fully protects against it.
Plastin 3 was first identified as a modifier of SMA because very high levels were found in cells from unaffected females lacking the SMN1 gene, despite them having the same levels of SMN protein as their SMA-affected siblings.
Plastin 3 plays an important role in the stabilisation of actin. Actin is a vital structural protein that has important functions in cell mobility, shape, and division. It forms a part of the cytoskeleton, which is the scaffolding, or indeed skeleton, that gives cells their shape and allows them to move.
Individual actin molecules are known as G-actin (globular actin), and these can bind together, or polymerise, to form F-actin (filamentous actin) (Figure A). F-actin polymers can then be linked together into bundles or networks that provide greater mechanical support for the cell, and Plastin 3 is known to help this process (Figure B).
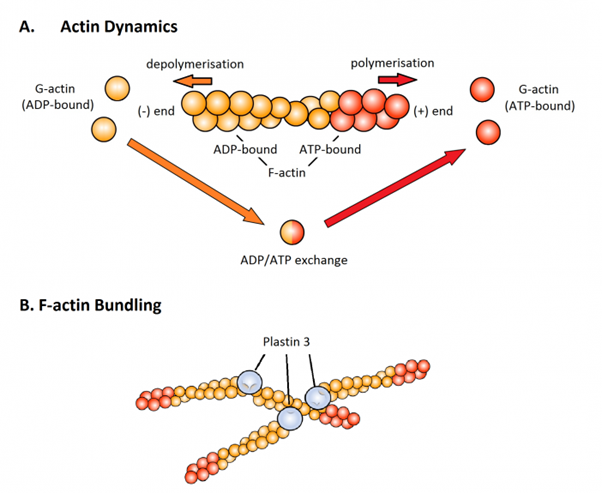
Figure. Plastin 3 is an F-actin bundling protein. (A) Energy-, or ATP-bound, G-actin molecules (separate red circles) are able to polymerise to form F-actin. This binding occurs at one end of the F-actin polymer known as the (+) end. Actin molecules bound to ATP are stable, but when ATP is converted to ADP, in a process that releases energy, the actin becomes unstable. When this happens in the middle of the F-actin molecule, the F-actin subunits are stabilised by their neighbours, but when this happens at the (-) end of the polymer, ADP-bound G-actin molecules (separate orange circles) are released. The ADP bound to the free G-actin can then be converted to ATP, in a process requiring energy, so that the cycle can continue. This process of actin assembly and disassembly, allows cells to dynamically alter the structure of their cytoskeletons. (B) Plastin 3 is involved in the bundling together of multiple F-actin molecules to form networks of F-actin, providing additional mechanical support.
The Jennifer Trust (now SMA UK) recently reported on a follow up study from Christine Beattie’s group, which showed that experimentally increasing Plastin 3 levels in zebrafish with low SMN levels improved neuromuscular junction and motor function defects.
The Wirth group has now published their latest research on Plastin 3, this time making use of mouse models of SMA in order to better understand the mechanism of Plastin 3-mediated protection against the disease.
The researchers experimentally increased the levels of Plastin 3 in SMA model mice that usually have a reduced lifespan and display defects in the structure and function of their neuromuscular junctions.
Plastin 3 overexpression in all cells of the body was able to improve a number of structural defects seen in the lower motor neurons and muscles of SMA mice. Motor neuron cell body size was increased, the number of synaptic vesicles containing neurotransmitter was partially restored, the stability of axons was improved, denervation was less pronounced, and muscle fibre size was enhanced.
Expression of Plastin 3 in the motor neurons alone also increased muscle fibre size, suggesting that the improvements in lower motor neuron structure that lead to the observed improvement in function, i.e. neurotransmission, likely account for the increased muscle fibre size.
These improvements in Plastin 3-overexpressing SMA mice resulted in a partial rescue of motor function, and a moderate increase in survival in less severe SMA mice.
The positive effects of Plastin 3 upregulation were not due to an increase in SMN levels. Furthermore, the natural levels of Plastin 3 were unaltered in SMA mice, contrasting with the downregulation of Plastin 3 seen in SMA zebrafish.
Together these results suggest that the protective effects of Plastin 3 are not dependent on the levels of SMN protein. Instead, it appears that overexpression of Plastin 3 increases the amount of F-actin in the lower motor nerves, which in turn improves axon stability through affecting actin and cytoskeleton dynamics. This indicates that Plastin 3 may be a therapeutic target for any disease that affects the motor neuron.
In summary, work from human cells, zebrafish, and now mice highlights the potential of Plastin 3 as a therapeutic target for SMA. Drugs able to increase Plastin 3 protein levels may prove beneficial to SMA and other motor neuron disease patients.
Further Information
The Abstract from the Latest Wirth Laboratory Plastin 3 Research Paper >
