SMN Gene Therapy in a Large SMA Model
SMN Gene Therapy in a Large SMA Model
Page last updated: 6th January 2015
The mouse is the most frequently used model organism for testing therapies that have the potential to treat human disease. Nevertheless, a significant proportion of drugs that provide benefit to mouse models of disease, fail in costly and time-consuming human clinical trials. Considerable differences in the make-up and genetics of mice and humans are the likely cause, for instance blood-brain-barrier drug permeability.
Non-human primates have also been used in pre-clinical experiments. Since they are more closely related to humans than mice are to humans, results obtained in non-human primates are more likely to accurately predict what will happen in humans. However, prohibitive costs and time-scales, and ethical considerations often mean that non-human primates are not used.
Prof. Arthur Burghes (Ohio State University, Ohio, USA) and colleagues have just published a study aimed at determining whether reducing SMN levels in pig motor neurons results in symptoms similar to SMA. This would potentially produce a large animal model for the disease, which could prove useful for pre-clinical testing of therapies.
To generate a pig SMA model, the researchers used the same type of virus that has been used to restore SMN, and that is currently in Phase I clinical trials. However, in this instance, rather than increasing SMN protein levels in cells and tissues, the viruses were engineered to target the pig SMN gene to reduce the amount of SMN protein it produces (See Figure).
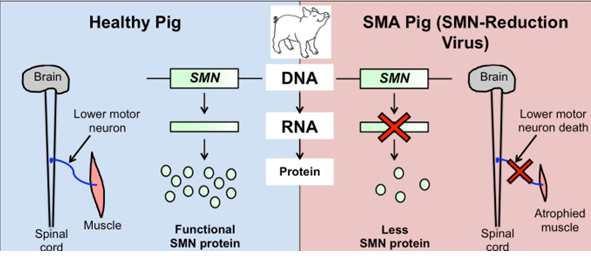
Figure. A large animal model for SMA. Genetically engineered viruses that target the pig SMN gene for reduction can be injected into pigs. These viruses travel around the body and produce a molecule that is able to decrease the amount of SMN protein. This causes death of the lower motor neurons and SMA-like symptoms.
Injection of these viruses into the spinal cord of five-day old pigs was shown to reduce SMN protein levels and caused key SMA-like features, for example muscle weakness, abnormal posture, and a loss of motor neurons.
The pig model was then used to see whether these SMA-like symptoms could be corrected using SMN gene therapy.
One day after the SMN-reduction virus was injected, the SMN gene therapy was administered. Of four treated animals, one displayed only mild weakness, while the remaining three were unaffected.
In order to determine if the gene therapy was useful at post-symptomatic stages, pigs were given the SMN gene therapy about four weeks after the SMN-reduction virus – at a time when SMA-related symptoms were present. Importantly, these later administrations of the gene therapy had a marked impact on disease progression.
This work from the Burghes Laboratory indicates that the virus-mediated SMN gene therapy is able to improve symptoms in a large animal model of SMA, which is more closely related to humans than mice are to humans.
It also suggests that the therapy may be able to positively affect disease progression, even when given after symptoms have started. This new model for SMA is likely to be a useful tool for assessment of therapies with potential to treat SMA.