New Highly Selective SMN2 Modifying Drugs Identified
New Highly Selective SMN2 Modifying Drugs Identified
Page last updated 13th August 2014
Researchers in the US have identified three new, orally available compounds that efficiently and selectively target the SMN2 “backup” gene leading to an increase in SMN protein levels.
The SMN1 gene provides all the SMN protein that we require, while SMN2 usually produces only a fraction of that (Figure 1). SMA patients lack SMN1 and rely only on SMN2 for their SMN protein. The drugs identified in this study, which was published in the prestigious journal Science, are able to affect the processing of SMN2, such that much more SMN protein is made.
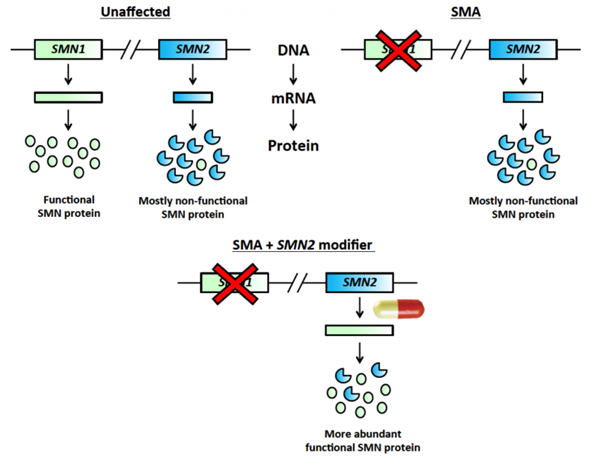
Figure 1. Newly identified modifier drugs specifically target the SMN2 gene, leading to increased production of the SMN protein.
Unlike other small molecule drugs that have been previously shown to affect SMN2 and the amount of SMN protein it can make, these new compounds appear to be much more selective for the SMN2 gene – they alter only a handful of other genes.
This drug specificity is vital to prevent unwanted and often dangerous side effects, and is the basis of antisense-oligonucleotide gene therapy, which is also showing great promise for SMA (ENDEAR trial).
The compounds, which have been named SMN-C1, SMN-C2, and SMN-C3, were first identified in a chemical screen designed to identify drugs able to up-regulate SMN protein production from SMN2. The drugs were all then shown to increase SMN levels in SMA patient skin and nerve cells derived from induced pluripotent stem (iPS) cells.
Mouse models of SMA were subsequently used to assess drug efficacy in live mammals. The compounds were shown to drastically increase SMN protein levels in all tissues tested, including the brain and various different muscles, even after a single dose.
This increase in SMN levels resulted in drastic improvements in neuromuscular junction integrity, lower motor neuron health, motor function, and overall survival. The effects of these compounds are the best ever reported for a small molecule drug not specifically designed for the purpose of treating SMA.
Importantly, the compounds worked at very low concentrations, showed responses dependent upon their concentrations, and are able to be taken in tablet form.
It is currently unknown exactly how the SMN2 modifying compounds exert their effects, but future work is planned to clarify the molecular basis of their specificity for the backup gene.
Given their efficacy, specificity, and oral availability, these compounds are undoubtedly an exciting possible future therapy for SMA patients. The lead compound has been shown to be safe and well tolerated in healthy volunteers, and clinical trials in SMA patients are planned for the near future.
