Novel Antisense Therapy Removes the Brakes on SMN Protein Production
Novel Antisense Therapy Removes the Brakes on SMN Protein Production
Page last updated: 11th January 2017
Dr James Sleigh our Scientific Research Correspondent reports on a potential new therapy for SMA, which could possibly be combined with other drugs such as Spinraza to enhance clinical effectiveness, has been identified and successfully tested in mouse models of the disease:
Research performed in the laboratory of Charlotte Sumner at Johns Hopkins University in the USA, has identified a small stretch of human DNA, which produces a molecule called SMN-AS1. SMN-AS1 is capable of restricting how much SMN protein is made by the SMN genes.
SMN-AS1 is found in abundance in the central nervous system (brain and spinal cord) of both mice and humans, and its presence appears to inversely correlate with the availability of SMN protein. This means that the more SMN-AS1 is present, the less SMN protein is found. This occurs because SMN-AS1 attracts important additional molecules to the SMN genes, preventing them from freely producing SMN protein.
Given this role of SMN-AS1 in limiting SMN protein production, the researchers had the idea that artificially reducing SMN-AS1 levels may partially remove the brakes on SMN protein expression.
To test this idea, antisense oligonucleotides (ASOs) targeting SMN-AS1 were designed. In contrast to the ASO Spinraza, which alters SMN2 splicing to increase SMN protein production, the SMN-AS1 ASO was devised to destroy the SMN-AS1 molecule.
Reducing the levels of SMN-AS1 in patient-derived cells and mouse nerve cells in culture was shown to increase the availability of the SMN protein. Given these successful results, the researchers went on to test the ASO targeting SMN-AS1 in a mouse model of SMA. When given to SMA mice, the drug decreased SMN-AS1 levels in the brain and spinal cord, leading to an increase in SMN protein expression. Unfortunately, this had little to no impact on the symptoms and survival of these animals.
However, when given in combination with low doses of an ASO targeting SMN2 (very similar to Spinraza), it provided a greater benefit than when the SMN2 ASO was administered alone. SMA mice receiving this combination therapy showed a larger increase in SMN protein production, and greater improvements in motor function, weight gain, and, most importantly, survival.
That is why this new ASO targeting SMN-AS1 is exciting; by relieving the brakes on SMN2 expression, i.e. increasing the availability of SMN2 messenger RNA, the SMN-AS1 ASO potentially provides Spinraza with more material to work with resulting in greater increases in SMN protein (See Figures 1 and 2).
Targeting SMN-AS1 for degradation is a potential therapeutic strategy for SMA.
Figure 1:
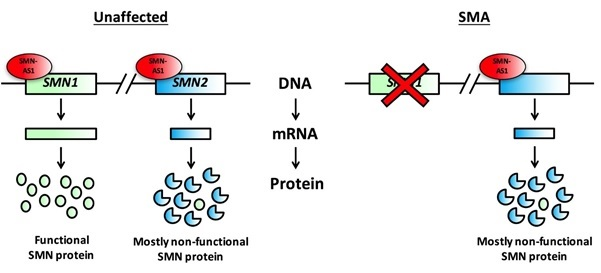
Figure 1. People unaffected by SMA possess two SMN genes; SMN1 (green rectangle) produces all the functional SMN protein required for a healthy nervous system, while SMN2 (blue rectangle) only produces a fraction of that (top left). SMN-AS1 (red oval) is a naturally occurring molecule in the body that restricts the amount of SMN protein made from both SMN genes. SMA patients lack a functioning SMN1 gene and rely solely on SMN2 for the production of SMN protein, meaning that they have much lower levels of functional SMN Protein (top right).
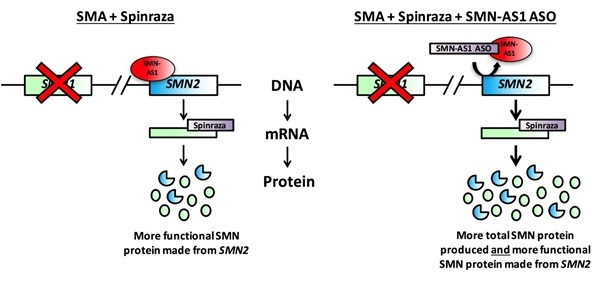
Figure 2:
Figure 2. Spinraza (purple rectangle) is an antisense oligonucleotide (ASO) that specifically recognises the messenger RNA (mRNA) produced from the SMN2 gene (top left). The SMN2 mRNA is processed in a different manner when Spinraza is present, resulting in the production of more functional SMN protein from this backup SMN gene. The new therapeutic strategy identified by the Sumner laboratory uses an ASO to remove the SMN-AS1 molecule from the SMN2 gene, allowing the gene to produce greater quantities of mRNA (top right).
This gives Spinraza more material to work with, and produces larger amounts of functional SMN protein than when Spinraza is administered on its own.
Although only at the pre-clinical stage, this research indicates that SMN-AS1 is a clinically relevant therapeutic target for SMA, and that combining other drugs with Spinraza may have additional therapeutic benefit. It should, however, be noted that such combination therapy approaches are likely to encounter new challenges in clinical development.