The Genetics of 5q Spinal Muscular Atrophy
The Genetics of 5q Spinal Muscular Atrophy
You can:
- Download a printed pdf version of this information here >
- Ask us to send you a printed version: publications order form >
Who this is for
Many people affected by Spinal Muscular Atrophy (SMA) want to know more about what a diagnosis of SMA means for other members of the family. Some want to know what it may mean for possible future pregnancies.
This guide covers the genetics of 5q SMA. This includes childhood onset SMA Types 1, 2 and 3 and adult onset SMA Type 4:
- How genetic conditions are caused
- The genes linked to 5q SMA
- How the genes are inherited
- The chances of SMA being inherited in different families
- Other rarer ways that 5q SMA can happen
- Genetic counselling
- Frequently asked questions
Some people find this guide ‘too science heavy’ and prefer our:
Summary of the Genetics of 5q SMA > .
There are other rarer forms of SMA with other genetic causes >
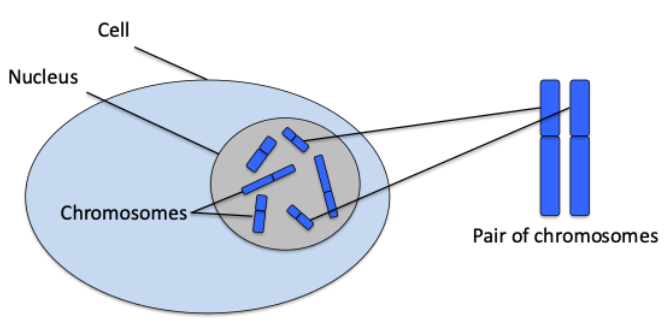
Genetic conditions are caused by alterations in our genes which prevent the gene from working properly.
Our bodies are made up of many millions of cells. Nearly all cells have a structure called the nucleus, which contains chromosomes that contain genes.
Chromosomes are compact bundles of DNA. (See Box 1 for an explanation of DNA.)
A gene is a specific section of DNA.
Body cells have two copies of each chromosome, as we inherit one copy from each parent.
We all have 46 chromosomes in each cell in our body, arranged in 23 pairs. There are two exceptions to this. Our red blood cells do not have any chromosomes. And our egg and sperm cells only have one of each pair of 23 chromosomes.
This means we have two copies of every gene (except in the egg and sperm cells where there is only one copy).
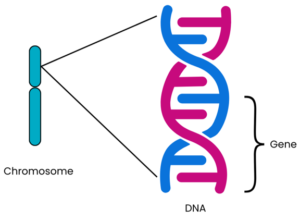
Genes are a sequence of code (called base pairs) that are read by the body to make proteins (see Box 1 for more information). Our cells need proteins for their structure, survival and to work correctly. We each have approximately 20,000 different genes making different proteins in our bodies¹‾². Each protein is made by a different gene and has its own unique function. The structure of the protein, and therefore its function, is determined by the order in which the base pairs are arranged in that particular gene. Usually, there are two copies of each gene on each chromosome pair: one inherited from each parent.
Sometimes a gene can contain an alteration known as a mutation. Genetic conditions occur when a mutation within a gene affects how the protein in our bodies is produced and how it works.
Box 1 – an explanation of DNA
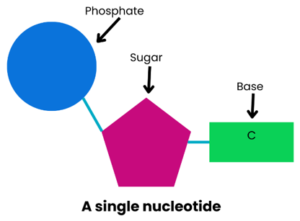
DNA is often described as a recipe book, or a set of instructions, because it contains the information needed for a person to grow and develop.
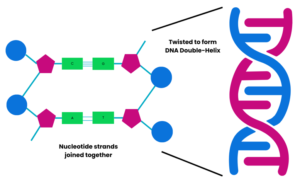
DNA is made up of lots of nucleotides joined together. Each nucleotide contains a phosphate, a sugar and a base. The phosphate and sugar are always the same but the base varies in each nucleotide. The base can be one of four: adenine (A), guanine (G), cytosine (C), or thymine (T).
These bases pair up: A with T and C with G. The order in which these pairs of bases are arranged affects how the ‘recipe book’ information is read. The joined base pairs hold the nucleotides together in strands that twist together to form the DNA double-helix shape.
There are two main genes involved: the SMN1 gene and the SMN2 gene.
-
The SMN1 gene3
Most people have two copies of the SMN1 gene. The SMN1 gene is essential for making a protein known as survival motor neuron protein (SMN protein). The SMN1 protein is crucial for the survival and function of lower motor neurons. These are the cells in the spinal cord and brainstem that send signals to the muscles. People with 5q SMA have two copies of the SMN1 gene that do not work properly. This means they are unable to produce enough SMN protein to have healthy lower motor neurons.
As long as you have one copy of the SMN1 gene that works properly, then enough SMN protein is produced in the body, and the nerves and muscles in the body will be fully functional.
-
The SMN2 gene3
A second gene also has a role in producing SMN protein. This is the Survival Motor Neuron 2 (SMN2) gene. It is sometimes referred to as the SMA “back-up gene”.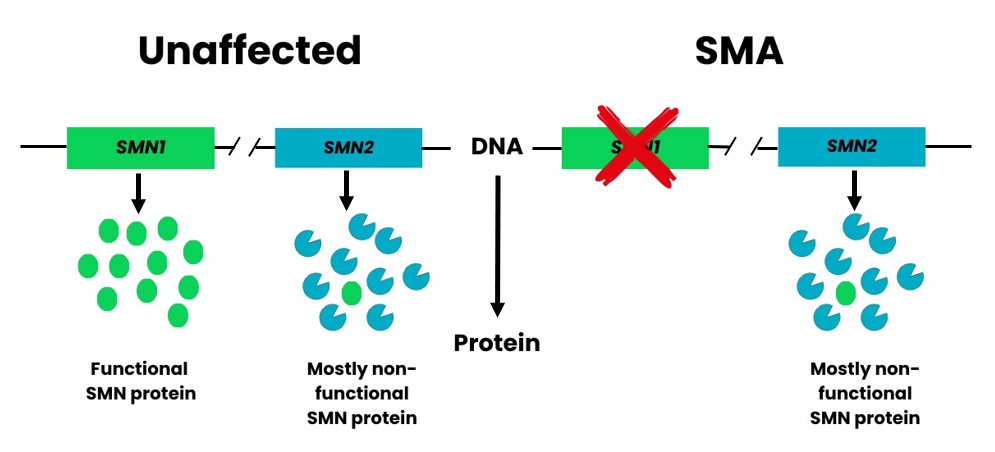
Figure 1. People possess two genes able to produce SMN protein. SMN1 produces all the functional SMN protein we need and is the gene affected in SMA. SMN2 only makes a small fraction of functional protein (about 10%). The large majority (about 90%) of protein produced from SMN2 is lacking an essential part and is consequently non-functional. Figure adapted from Burghes, A.H. and Beattie, C.E4.
A person with type 1 SMA typically has 1 or 2 copies of the SMN2 gene, whereas people with type 2 and type 3 have more copies of the SMN2 gene.
We all have 46 chromosomes in each cell in our body, except for egg and sperm cells. These are arranged in 23 pairs:
- 22 of the pairs are non-sex chromosomes, known as autosomes. These are found in both males and females.
- The 23rd pair consists of two sex chromosomes, which determine your sex. Females usually have two X chromosomes (XX), and males an X and a Y chromosome (XY).
An autosomal condition means that the gene alteration causing the condition is located on one of the autosomal chromosomes. It is not on one of the two sex chromosomes.
A recessive condition means that both of the pair of genes are altered.
5q SMA is an autosomal recessive condition
As 5qSMA is an autosomal recessive condition:
- a person will only have SMA if they inherit two altered copies of the SMN1 gene
- a person who carries one altered copy of the gene and one healthy copy will not have the condition but is a carrier. They do not have any symptoms, but the altered gene can be passed on to their children.
The SMN1 genes that are inherited and cause 5q SMA vary in the way they are altered. There are two main types of changes: Deletions and Point Mutations.
-
Deletion
This describes a type of alteration when a small section of DNA is missing. When part or all of a gene is missing, it can no longer make healthy protein. Instead, a shorter, often less useful (less functional) protein is made. In some instances, no protein is made at all. Figure 2 shows how missing a small part of the gene code could make it not work properly.

Figure 2. Deletion and Point Mutations. Taken from Skirton. H. and Patch, C. (2009) Genetics for the Health Sciences. Oxford: Scion Publishing6.
-
Point mutation
This is when a single base (nucleotide) within the DNA is altered, rather than there being a deletion. It is most common for these people to inherit one SMN1 gene with a point mutation. The other inherited SMN1 gene will be the more common deletion alteration. Figure 2 shows how missing a small part of the gene code could make it not work properly.
The chances of your children being carriers or having 5q SMA will depend on whether you or your partner have 5q SMA or are carriers. The chances stay the same for each pregnancy. Having one child who has 5q SMA or is a carrier does not change the chances for any further children.
The following diagrams show what the chances are in different families. For easier reading we just say SMA.
For the purpose of the diagrams, a ‘non-carrier’ means a person who does not carry the altered gene and does not have SMA.
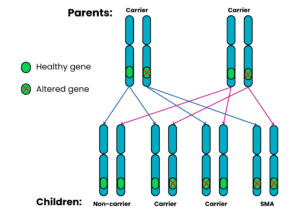
Autosomal recessive Family 1: Both parents are carriers
For each pregnancy, the chances are:
- Child will not have SMA and will not be a carrier: 1 in 4 chance (25%)
- Child will not have SMA but will be a carrier: 2 in 4 chance (50%)
- Child will have SMA: 1 in 4 chance (25%)
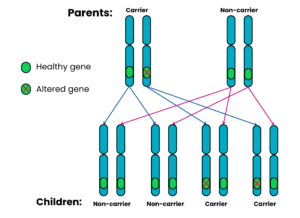
Autosomal recessive Family 2: One parent is a carrier, the other does not have SMA and is a non-carrier
For each pregnancy, the chances are:
- Child will have SMA: very low risk*
- Child will not have SMA and will not be a carrier: 2 in 4 chance (50%)
- Child will not have SMA but will be a carrier: 2 in 4 chance (50%)
- Carrier testing for someone with no family history of SMA would usually just cover SMN1 deletions. It would not check for a point mutation.
- A person can be reported as a non-carrier but there is still a small chance of them carrying a rarer (point) mutation.
- There is a small chance of a de novo mutation on the other copy of SMN1, so the risk is never zero. De novo means newly occurring, rather than being inherited. For more information see tab 6.
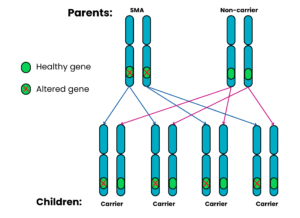
Autosomal recessive Family 3: One parent has SMA, the other does not have SMA and is a non-carrier
For each pregnancy, the chances are:
- Child will have SMA: very low risk*
- Child will not have SMA and will not be a carrier: not possible
- Child will not have SMA but will be a carrier: 4 in 4 chance (100%)
- Carrier testing for someone with no family history of SMA would usually just cover SMN1 deletions. It would not check for a point mutation.
- A person can be reported as a non-carrier but there is still a small chance of them carrying a rarer (point) mutation.
- There is a small chance of a de novo mutation* on the other copy of SMN1, so the risk is never zero. De novo means newly occurring, rather than being inherited. For more information see tab 6.
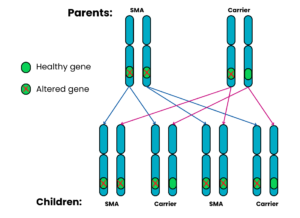
Autosomal recessive Family 4: One parent has SMA, the other is a carrier
For each pregnancy, the chances are:
- Child will not have SMA and will not be a carrier: not possible
- Child will have SMA: 2 in 4 chance (50%)
- Child will not have SMA but will be a carrier: 2 in 4 chance (50%)
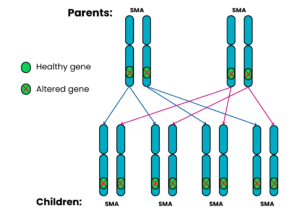
Autosomal recessive Family 5: Both parents have SMA
For each pregnancy, the chances are:
- All the children will have SMA (100%)
This can happen because the SMN1 alteration is:
- new in the affected person or
- not clearly present in the cells analysed in the carrier test.
This situation can be caused in one of the following ways:
-
De Novo
De novo means “from the beginning”. It is a type of alteration that occurs either
- when an individual sperm or egg is made, or
- when a sperm fertilises an egg, or
- when cells are dividing after fertilisation.
The most likely reason is an error in the making of the sperm or egg cell. As it occurs at this stage, the alteration will not be detected in either of the parents.
-
Germline Mosaicism
This is when an individual produces sperm or egg cells that differ in their genetics from all the other cell types in the body of that person. If the SMN1 gene is affected in this way, the sperm or eggs of that individual can have an altered SMN1 gene. This would go undetected by the carrier test.
-
Silent Carriers of 5q SMA
Another rare situation involves a carrier of SMA who has two or more SMN1 copies on one chromosome but none on the other. In these cases, the standard laboratory test may not be able to detect that the person carries a deletion within the SMN1 gene on the other chromosome in the pair. However, it may be possible for the genetics laboratory to work this out, by testing other family members or by doing further testing.
These rare scenarios can have implications for the chance of 5q SMA affecting a future pregnancy. They show that carrier testing for genetic conditions will never be 100% accurate. They highlight the importance of having genetic counselling specific to your own circumstances.
The laboratory and your clinical and genetics team can discuss any limitations of the testing with you. They will also try to ensure the testing for your family is as accurate as possible.
Genetic counselling is with a healthcare professional who has expert training in genetics. They will aim to explain results from your genetic testing in an easily understandable way. They will answer any questions you might have about the genetic aspects of the diagnosis.
People often discuss:
- implications or options for a future pregnancy
- if the diagnosis should be discussed with other family members who might wish to seek genetic counselling.
Q1. What is the waiting time for a genetics appointment?
A: You will usually be offered an NHS Genetics testing appointment within 18 weeks. However, you will be seen much sooner (usually within a few days) if you are currently pregnant and have a family history of SMA.
Q2. How long will it take to get the results of a genetic test for 5q SMA?
A: It can take several weeks before a result is returned. However, in most instances when SMA is being considered as a likely diagnosis it will be available much sooner. The clinical teams and laboratories try to make sure that it is available within 5 days for those suspected of having SMA Type 1.
Q3. I have no family history of 5q SMA, can I still be tested?
A: NHS Genetic testing is not usually available to people with no personal family history or connection to 5q SMA.
Q4. Can I have a genetic test for 5q SMA without having genetic counselling?
A: This is generally not possible via the NHS. Genetic counselling will give you the most up-to-date and accurate information about the implications of your test result and the options available to you.
Q1. A member of my family has been diagnosed with 5q SMA. I am pregnant and I do not know if I am a carrier. How do I get a quick referral to genetic services?
A: Contact your midwife or G.P. or obstetrician about what to do next and how to ask for an urgent genetic counselling referral. If this is not possible, you can contact your local genetic service directly. There is a list of centres on the Genetic Alliance UK website > You and your partner may be offered testing.
Q1. My partner is a carrier of SMA. We are thinking of having children. Where can I get tested to see if I am a carrier too?
A: Ask your G.P. to refer you to your regional clinical genetics centre. The main genetics clinics are usually in large cities, but outreach clinics may be held in other smaller hospitals across the region. You may also be offered a phone or virtual appointment.
Q2. There is a history of 5q SMA in my family. When should my partner and I have genetic testing?
A: Genetic counselling before pregnancy will give you and your partner more time to think about genetic testing and the possibly difficult decisions this can raise. You can still seek genetic counselling if you are already pregnant. Make sure to say you are pregnant and ask to be seen urgently.
Q3. We do not have SMA ourselves but have had one child with SMA. How can we find out if our next child will also have SMA?
A: It is most likely that you and your partner are both carriers of the gene alteration that causes 5q SMA. Genetic counselling is important so that you can get advice specific to your circumstances and consider your future options.
If you have another pregnancy together, the chance that your next child will have 5q SMA is likely to be 1 in 4 (25%). This is shown in the Family 1 diagram above. The copy of each gene inherited from each parent is random and cannot be predicted. Some couples who are both carriers decide to take that chance. Others want to consider alternative options when having children.
You can also discuss what is possible with the healthcare professionals involved. They should be able to help you make this very personal decision.
Q1. In a family with 5q SMA, who will be able to have genetic testing?
A: Your regional Genetics Centre > can give you specific advice about who might need to be tested. Close family members will be seen first to identify who might be carriers. The Genetics Centre staff might work with you to draw a family tree.
Q2. I am a carrier, should I suggest that other family members get tested?
A: Genes are inherited from parents and passed on from generation to generation. You share many of your genes with members of your extended family. It is therefore possible that your blood relatives may also be carriers of the same gene alteration.
You might want to tell your relatives you are a carrier. This will give them the option of asking for genetic counselling to obtain more information. They may then choose to have carrier testing. This can be particularly relevant if they are considering a future pregnancy.
Q3. My daughter has been diagnosed with 5q SMA. I am worried that her brother and sister might develop 5q SMA too. Should they be tested?
A: It is important for you to discuss this with the healthcare professionals involved and your family. Your decision may be influenced by the Type of SMA your daughter has. It could also be affected by any concerns you have about the health of your other children.
Q4. My sister’s son has been diagnosed with 5q SMA. I have a 4-year-old daughter. I am worried that she might develop SMA too. Should I have her tested?
A: You could request carrier testing at a Genetics Centre > to see whether you are a carrier of 5q SMA. After you get your result, talk to your healthcare team and family about testing your daughter.
Genetics centres do not always offer carrier testing in childhood. It removes the child’s right to make an informed decision when they are older.
Q5. For the last 20 years or so, I have had weakness and difficulties. More recently my mobility has deteriorated further. Our grandchild has recently been diagnosed with SMA Type 3. I now wonder if I might have SMA too. Can I request genetic testing?
A: There is a possibility that you might have SMA. You can ask your G.P. to refer you to a neurologist, who can review your symptoms. Your clinical signs and family history will be reviewed. You may be offered genetic testing for SMA. They may also advise whether they think your symptoms have a different cause.
Q6. We have SMA on my side of our extended family, but I have not had carrier testing. My children are now young adults. I have been told by my genetics centre that, because I am not planning to have any more children myself, I do not now need carrier testing now. What do you advise about this?
A: If a person is referred to clinical genetics with a family history of SMA, the genetics team would review the family tree. They would decide which relatives would benefit from genetic testing. They will discuss this decision with you and may suggest that other relatives are tested before you have testing yourself.
Q7. Two generations ago, my grandma’s first child died. From what I’ve read about SMA, I think the child might have had SMA Type 1. There was no genetic confirmation, so I understand I may not be eligible for NHS carrier testing. Can I have testing privately?
A: It is always preferable to use NHS services where possible. This ensures high quality testing and advice by qualified scientists and genetic services.
We are not able to recommend any specific private health services in the UK. If private testing is the only option, look for private providers able to match the following level of registration or accreditation:
- NHS laboratories are accredited by UKAS. Their website lists all of the molecular genetic labs > accredited in this way.
- NHS doctors are registered on the GMC register >.
- NHS genetic counsellors are registered with:
Q1. I have been tested for 5q SMA and the test has come back negative, but my consultant still thinks I have SMA. Is this possible?
A: In a small number of cases, the genetic basis is more complex. Further genetic and / or other testing may be necessary. Your doctor will advise you depending on your symptoms and the tests you have had so far.
Q2. My son has SMA symptoms, but the test has come back negative. Is it possible that he has SMA?
A: Routine testing for SMA will confirm the diagnosis in most people. Sometimes further genetic and / or other testing may be needed. Your doctor will advise you depending on your son’s symptoms and the tests he has had so far. This may include investigations for other conditions that can present in a similar way to SMA.
Genetics and Genetic Testing:
Phone: 0207 704 3141
Support:
Phone 01789 267520.
- International Human Genome Sequencing Consortium (2004) ‘Finishing the euchromatic sequence of the human genome’, Nature, 431, pp. 931-945.
- Pennisi, E. (2012) ‘ENCODE project writes eulogy for junk DNA’, Science, 337(6099), pp. 1159-1161.
- Lefebvre S et al. (1995) Identification and characterization of a spinal muscular atrophy-determining gene. Cell 80: 155-165.
- Burghes, A.H. and Beattie, C.E. (2009) ‘Spinal muscular atrophy: why do low levels of survival motor neuron protein make motor neurons sick?’ Nature Reviews Neuroscience, 10, pp. 597-609.
- Verhaart IEC, Robertson A, Wilson IJ, Aartsma-Rus A, Cameron S, Jones CC, Cook SF, Lochmüller H (2017) Prevalence, incidence and carrier frequency of 5q–linked spinal muscular atrophy –a literature review. Orphanet J Rare Dis 12: 124.
- Skirton. H. and Patch, C. (2009) Genetics for the Health Sciences. Oxford: Scion Publishing.
Was this page useful?
 Version 8
Version 8
Author: SMA UK Information Production Team
Last updated: July 2025
Next full review due: July 2028
Links last checked: July 2025
This page, and its links, provide information. This is meant to support, not replace, clinical and professional care.
Find out more about how we produce our information >.
