What is 5q Spinal Muscular Atrophy?
What is 5q Spinal Muscular Atrophy?
Spinal Muscular Atrophy (SMA) is a rare, neuromuscular condition. It causes progressive muscle wasting (atrophy) and weakness. It may affect crawling and walking ability, arm, hand, head and neck movement, breathing and swallowing.
There are several forms of SMA that have different genetic causes and a wide spectrum of how severely children and adults are affected. The most common form is known as ‘5q SMA’; the term ‘5q’ refers to its genetic cause and includes SMA Types 1, 2, 3 and 4.
For information about some of the rarer forms of SMA that have different genetic (non-5q SMA) causes, see: Rarer Forms of SMA.
-
The SMN1 gene2
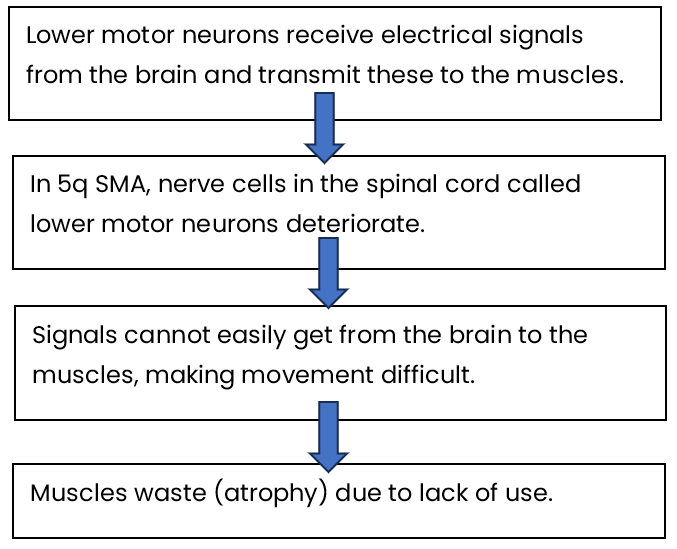
5q SMA affects the nerve cells called lower motor neurons.
Lower motor neurons are found within the spinal cord. They receive electrical signals from the brain and carry these to the muscles. These respond, leading to movement – such as crawling and walking. In the same way, the lower motor neurons provide signals to the muscles we use for breathing and swallowing.
For these nerve cells to be healthy, our Survival Motor Neuron 1 (SMN1) genes6 must produce enough Survival Motor Neuron (SMN) protein.
The SMN1 gene is on chromosome 5 in the region labelled ‘q’. This is why this form of SMA is called ‘5q SMA’.
For more information, see: The Genetics of 5q SMA.
Most people have two copies of the SMN1 gene. People with 5q SMA have two altered copies of the SMN1 gene. This means they are unable to produce enough functional (working) SMN protein to have healthy lower motor neurons7. As a result, the lower motor neuron cells in the spinal cord deteriorate. This means signals cannot easily get from the brain to the muscles making movement difficult. The muscles then waste due to lack of use – this is known as muscular atrophy.
In summary:
- The SMN2 gene²
A second gene also has a role in producing SMN protein. This is the Survival Motor Neuron 2 gene (SMN2), sometimes referred to as the SMA ‘back-up’ gene.
However, it is estimated that only about 10% of the SMN protein made from SMN2 is able to work properly. The large majority – about 90% – of protein produced from SMN2 is lacking an essential part meaning that it does not work8.
So, while SMN2 can make some functional SMN protein, it cannot produce enough to fully make up for the altered SMN1 gene in people who have 5q SMA.
Unlike most genes, the number of copies of SMN2 on each chromosome can vary from one person to the next9; there can be between 0 – 8 copies.
If you look across the whole population of people who have 5q SMA, the severity of SMA is linked to how much SMN protein is made9,10. This means there is a general relationship between the number of SMN2 copies (‘SMN2 copy number’) and the likely severity of SMA symptoms9,10.
Having more SMN2 copies is generally associated with less severe SMA symptoms. However, at an individual level, a person’s SMN2 copy number on its own does not give an accurate prediction about the Type or severity of their SMA3,4. This is likely to be because other genetic, and possibly environmental, factors have an influence on the condition.
Bearing this in mind, the following table gives a summary of:
- how many SMN2 copies the majority of people with each Type of SMA may have (see ‘usual number’ column), and
- the possible range of copy numbers that people with each Type of SMA may have (see ‘range’ column)
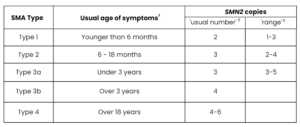
Table adapted from Tillmann et al. 201811
Before drug treatments (also known as ‘disease-modifying’ treatments) started to be developed and tested for 5q SMA, clinicians and researchers gathered information about what impact someone with SMA might expect the condition to have on them.
This is called the ‘natural history’ of the condition.
This led to 5q SMA being divided into four main Types of SMA: Types 1, 2, 3, and 4. Sometimes a baby is affected before birth; this is called Type 0.
These ‘Types’ of SMA were based on the age that symptoms began, and what physical milestone (e.g. sitting, standing, walking) could be achieved. It was agreed that there could be variation both within and between ‘Types’.
| SMA Type | Age symptoms usually begin | Motor milestones |
|---|---|---|
| Type 1 | 0-6 months | Unable to sit or roll independently |
| Type 2 | 7-18 months | Able to sit but not walk independently |
| Type 3 | 18 months – 18 years | Able to walk though may lose this ability over time |
| Type 4 | 18 years + | Mild walking difficulties |
*Adapted from Finkel et al., 20171
This classification system is still used for adults, teenagers and children living with SMA in the UK even though for many, care and treatment is changing the outcome of their SMA.
-
Due to symptoms of SMA
This classification system of ‘Type’ is also still used when a baby, child, young person or adult shows symptoms and SMA is confirmed via an SMN1 gene test.
Parents / carers will often have been concerned about symptoms of weakness in their child. Symptoms may have been noticed by doctors, the health visitor or community nurse.
Adults who are diagnosed with SMA Type 4 may have had concerns about their muscle weakness or fatigue.
A GP may have met few, or even no children or adults who have SMA, so should make an immediate referral to a Regional (Specialist) Neuromuscular Centre.
A paediatrician or neurologist will then examine any child or adult with suspected SMA and will also ask about their medical history and any concerns.
A blood sample is then taken for DNA (genetic) testing. This looks for a ‘deletion mutation’ in the Survival Motor Neuron 1 (SMN1) gene on chromosome 5.
The result of the SMN1 deletion test is usually available within 2 weeks though sometimes this may take longer.
At the same time, the number of copies of the SMN2 gene (the back-up gene for SMN1) is also given. Having more copies of the SMN2 gene is generally associated with less severe symptoms of SMA.
-
Due to newborn screening:
There is currently a newborn screening research study based in the Oxford / Thames Valley region. If your baby has been part of this, they will have had a newborn screening test that looks for the SMN1 gene deletion and the number of copies of the SMN2 gene. The number of SMN2 copies indicates what symptoms and effects the baby’s SMA would be likely to cause if they do not receive drug treatment and appropriate care. The baby may or may not be showing symptoms at this stage.
There is no cure for SMA and until late 2019 there were no NHS-approved drug treatments specifically for SMA in the UK.
There are now three NHS-funded, disease-modifying drug treatments:
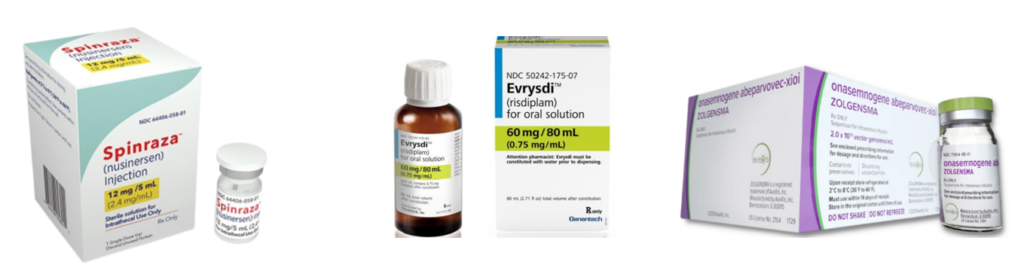
These treatments are not suitable for everyone who has 5q SMA, but most people in the UK who have SMA Type 1, 2 or 3 can receive one of them. At the moment there have been no clinical trials of any of the treatments with people who have SMA Type 4.
These drugs can change what motor milestones babies and children may be able to achieve and improve their general health. The treatments work best if started before there is any muscle weakness, or when this is minimal. It is therefore important for treatment to be started as soon as possible². This is why clinicians and patient groups are calling for the earliest possible introduction of newborn screening for SMA in the UK.
For adults living with SMA, drug treatment that can stabilise the condition later in life may also make a positive difference – for example, helping with fatigue or preventing the loss of the ability to use a finger to control a powerchair or laptop.
For summaries about these NHS-funded drug treatments, see:
Whatever the outcome, any of these drug treatments must be combined with the best supportive care and management of symptoms. Information, such as how someone’s breathing ability is affected by their SMA and whether they are able to sit, stand or walk, is very important when it comes to making decisions about a drug treatment. This was why in 2017, the ‘International Standards of Care for SMA’ (SoC)3,4,5 used the following broad categories to outline best care and support based on whether a child, young person or adult can be described as:
- A non-sitter – those who are unable to sit
- A sitter – those who are able to sit but not walk
- A walker – those who are able to walk
Though these guidelines are still important, they were written before the new drug treatments became more widely available. Nationally and internationally, clinicians, researchers and people living with SMA are working together to review and update these guidelines and keep up with the positive changes resulting from SMA therapies. Patient groups and clinicians in the UK want to see this work taking place here.
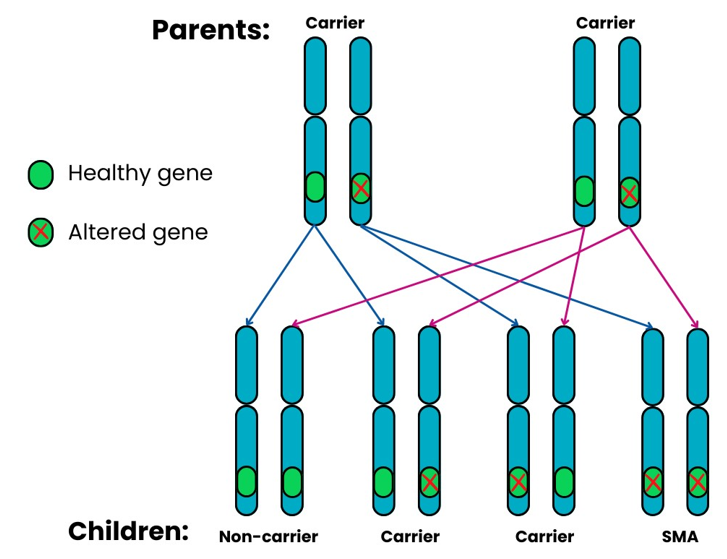
5q SMA is passed from parents to their children through altered SMN1 genes. It usually follows an autosomal, recessive pattern of inheritance. This means that:
When two SMA carriers have a child together, for each pregnancy there is a:
- 1 in 4 (25%) chance that the child will inherit both altered copies of the SMN1 gene and will have SMA.
- 2 in 4 (50%) chance that the child will inherit one altered copy and one healthy copy of the SMN1 gene and will be a carrier.
- 1 in 4 (25%) chance that the child will inherit two healthy copies of the SMN1 gene and will not be a carrier or have SMA.
There are a very small number of people who develop 5q SMA despite standard carrier tests showing that one or both parents are not carriers. For example, in around 2% of cases of 5q SMA, the ‘mutation’ (when part of the DNA changes or is missing) occurs for the first time in the affected person.
Even if a parent of a child with SMA is identified as a non-carrier, it is important that they still have genetic counselling. This will explore their own specific circumstances.
For more detailed information, please see: The Genetics of 5q SMA.
-
 An estimated 1 in 40 people carry the altered SMN1 gene12.
An estimated 1 in 40 people carry the altered SMN1 gene12.
This means that although SMA is a rare condition, around 1.69 million people13-15 in the UK are carriers of the altered SMN1 gene.
-
 Every month in the UK, 4 babies are born with 5q SMA.
Every month in the UK, 4 babies are born with 5q SMA.
This is the incidence of SMA – the number of new people diagnosed with SMA at any one time.
Pooling all published newborn screening data has shown that one in every 14,000 babies worldwide are born with a Type of 5q-linked SMA16, 17.
For every 100 who have SMA, approximately 60 babies would have the most severe SMA Type 1 (60%)18.
In the UK in 2022, there were 676,275 live births 19-21. This suggests that approximately 48 babies were born who will develop a Type of 5q SMA. About 29 babies (60%) would have the more severe SMA Type 1.
-
Global studies suggest that worldwide, between 1 and 2 people in every 100,000 have a Type of 5q SMA12.
 This is the prevalence – how many people are living with SMA in a population at any one time.
This is the prevalence – how many people are living with SMA in a population at any one time.
In mid-2022, the UK population was approximately 67.6 million13-15. The global studies therefore suggest that between 675 and 1,350 people have SMA in the UK at any one time. There is no central information source, so the exact number is unknown.
However, these global studies were published before drug treatments became available. These treatments have had a life-saving impact for many children who have SMA Type 1 and have led to life-changing, healthier lives for many others. It therefore seems reasonable to suggest that the SMA prevalent population will slowly increase year by year.
There is still a considerable amount of research into SMA taking place around the world, and there are more drugs being tested in clinical trials. This will continue to improve our understanding of SMA and the effects of, and responses people have to, the current treatments. It will also help with the further development of effective management and care.
To keep up-to-date with the latest developments in research and drug treatments, see Treatments and Research.
1. Finkel RS Finkel RS et al. (2017) ENMC SMA Workshop Study Group. 218th ENMC International Workshop: Revisiting the consensus on standards of care in SMA Naarden, The Netherlands, 19-21 February 2016. Neuromuscul Disord 27: 596-605.
2. Dangouloff T & Servais L (2019) Clinical evidence supporting early treatment of patients with spinal muscular atrophy: current perspectives. Ther Clin Risk Manag 15: 1153-1161.
3. A Guide to the 2017 International Standards of Care for SMA. Available at: smauk.org.uk/09nn (Last accessed: 25th July 2022).
4. Mercuri E et al. (2018) Diagnosis and management of spinal muscular atrophy: Part 1: recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord 28: 103-115.
5. Finkel RS et al. (2018) Diagnosis and management of spinal muscular atrophy: Part 2: Pulmonary and acute care; medications, supplements and immunizations; other organ systems; and ethics. Neuromuscul Disord 28: 197-207.
6. Lefebvre S et al. (1995) Identification and characterization of a spinal muscular atrophy-determining gene. Cell 80: 155-165.
7. Lunn MR & Wang CH (2008) Spinal muscular atrophy. Lancet 371: 2120-2133.
8. Ruggiu M et al. (2012) A role for SMN exon 7 splicing in the selective vulnerability of motor neurons in spinal muscular atrophy. Mol Cell Biol 32: 126-138.
9. Mailman MD et al. (2002). Molecular analysis of spinal muscular atrophy and modification of the phenotype by SMN2. Genet Med 4: 20-26.
10. Lefebvre S et al. (1997). Correlation between severity and SMN protein level in spinal muscular atrophy. Nat Genet 16: 265-269.
11. Tillmann R et al. (2018) Spinal Muscular Atrophy (SMA) type 1, a changing phenotype: implications for motor function and physiotherapy management from the Nusinersen Expanded Access Program (EAP). APCP Journal 9: 4-12.
12. Verhaart IEC et al. (2017) Prevalence, incidence and carrier frequency of 5q–linked spinal muscular atrophy – a literature review. Orphanet J Rare Dis 12: 124.
13. Office for National Statistics (November 2022) Population of England and Wales: tinyurl.com/ykftm77n (last accessed 27th November 2023)
14. Scotland’s Census 2022 – Rounded Population Estimates: tinyurl.com/2bfw635h (last accessed 27th November 2023)
15. 2022 Mid-Year population Estimates for Northern Ireland tinyurl.com/mpphmswh (last accessed 27th November 2023)
16. Aragon-Gawinska et al. (2023) Spinal muscular atrophy treatment in patients identified by newborn screening—A systematic review. Genes 14: 1377.
17. Belter et al (2024) Newborn Screening and Birth Prevalence for Spinal Muscular Atrophy in the US” in JAMA Pediatrics on July 15, 2024.
18. Verhaart IEC, et al. (2017) A multi-source approach to determine SMA incidence and research ready population. J Neurol 264: 1465-1473.
19. Office of National Statistics (2021) Births in England and Wales: 2020. Available at: tinyurl.com/3ky86caf (last accessed 13th November 2023)
20. Annual births, deaths, marriages and other vital events. Vital Events Reference Tables 2022. Section 3 Births. Published on 20th July 2023: tinyurl.com/4shymnbj (last accessed 13th November 2023)
21. Northern Ireland Statistics and Research Agency Registrar General Annual Report 2022 Births. Published: 26th October 2023. tinyurl.com/yc7j3msb (last accessed 13th November 2023)
 Version 9
Version 9
Author: SMA UK Information Production Team
Last updated: July 2024
Next full review due: November 2024
Links last checked: November 2023
The information provided in this guide, on our website, and through links to other websites, is designed to complement not be a substitute for clinical and professional care and advice.
For more detail about how we produce our information, please see these pages.
If you have any feedback about this information, please do let us know at: information@smauk.org.uk